Recalls
On this page you’ll find recent recall alerts for food and feed products distributed or produced in Georgia. These alerts include the reason for the recall, a description of the issue, and a complete listing of affected products with identifying information.
Learn more about recallsMoonlight Companies (10/27/2025)
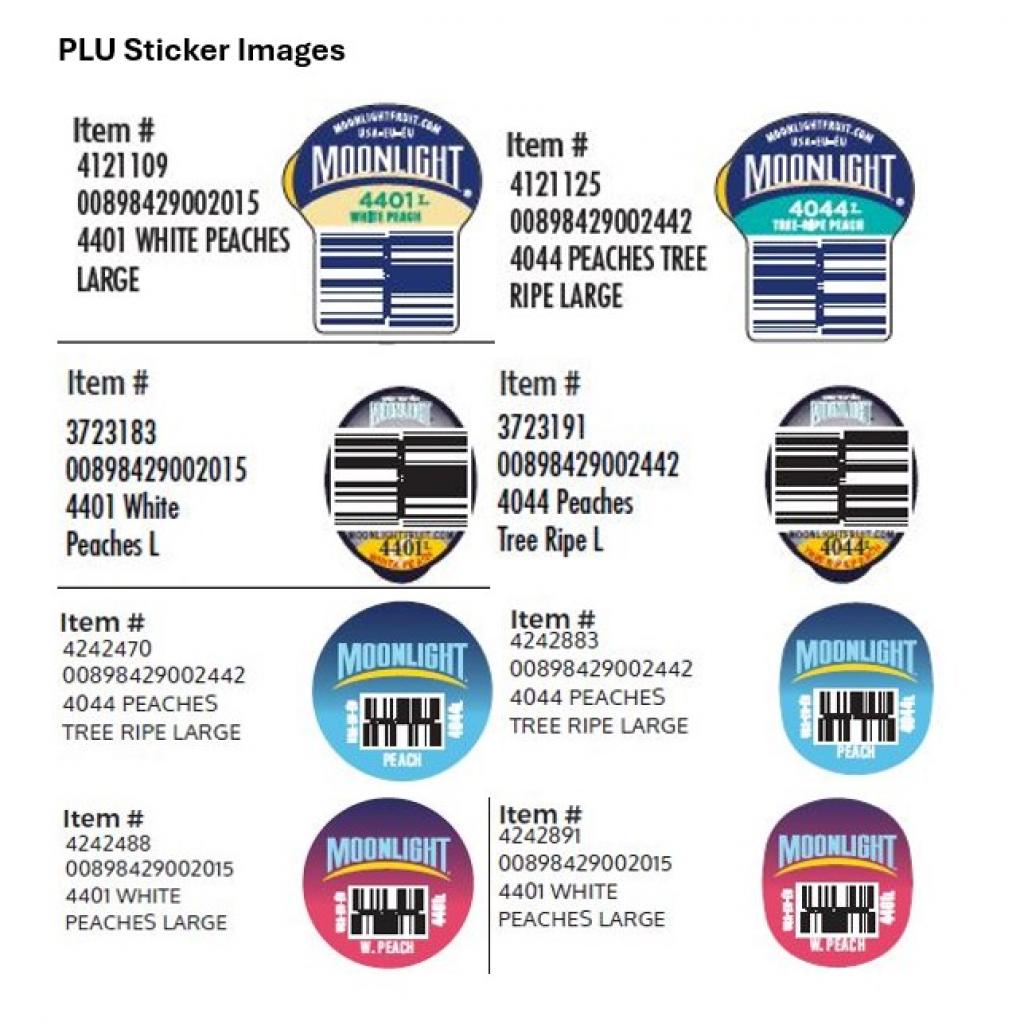
Moonlight Companies is voluntarily recalling California-grown conventional yellow and white peaches because they have the potential to be contaminated with Listeria monocytogenes, an organism that can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain, and diarrhea. Listeria infection can cause miscarriages and stillbirths among pregnant women.
The recalled peaches were sold at retail stores nationwide between September 16, 2025 and October 29, 2025. The peaches were either sold as individual pieces of fruit bearing PLU stickers or as multi-packs. This recall does not include packages or PLU stickers with the words “Washington” and/or “Organic.” The recalled products are listed in the following summary table and images are below:
| Product Description | Packaging Type | PLU Sticker | UPC | Facility Code | Lot Code |
|---|---|---|---|---|---|
| Moonlight Yellow Peaches | Individual pieces | 4401 4044 | P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT | |
| Multi-packs | 8 10248 03165 6 8 98429 00209 1 | P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT | ||
| Moonlight White Peaches | Individual pieces | 4401 4044 | P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT | |
| Multi-packs | 8 10248 03163 2 8 98429 00209 1 |
P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT | ||
| Moonlight White Peaches (“Peppermint Peach”) | Multi-packs | 8 98429 00266 4 8 10248 03163 2 8 10248 03087 1 8 10248 03186 1 |
P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT | |
| Kroger Yellow Peaches | Multi-packs | 11110 18174 | P1 | 01PCLC, 03PCAF, 106PCLF, 113PCAF, 113PCLF, 129PCLF, 134PCLF, 142PCLF, 150PCLF, 151PCLF, 159PCABA, 159PCABB, 159PCPG10, 20, 22PCAB, 22PCPG10A, 22PCPG10B, 22PCP8A, 22PCPG8B, 22PCPG8C, 23, 25, 30PCEN, 40LT, 40YP#3, 44PCLC, 44PCLCB, 45, 51PCLC, 51PCLCB, 86PCAF, 69PWPR, 79PWPRT |
This recall is being conducted because Listeria monocytogenes was identified in the packing facility environment.
No illnesses have been reported to date.
Consumers with questions can contact 855-215-5017, Monday – Friday from 8 am – 5 pm Eastern Time.
This recall is being carried out with the knowledge of the U.S. Food and Drug Administration.









New Hoque & Sons Inc. (10/27/2025)
New Hoque & Sons Inc. of Maspeth, NY, is recalling its packages of “Dry Ghoinnya Fish” because the product was found to be uneviscerated.
The recalled “Dry Ghoinnya Fish” were distributed nationwide in retail stores. The product comes in a 10-12 pound, clear plastic package marked with an expiration date of 5/19/25 stamped on the bottom. The product UPC code is 908172635412.
The recall was initiated after routine sampling by New York State Department of Agriculture and Markets Food Inspectors and subsequent analysis by Food Laboratory staff revealed the product was not properly eviscerated prior to processing.
The sale of uneviscerated fish is prohibited under New York State Agriculture and Markets regulations because Clostridium botulinum spores are more likely to be concentrated in the viscera than any other portion of the fish. Uneviscerated fish have been linked to outbreaks of botulism poisoning. Symptoms of botulism include dizziness, blurred or double vision and trouble with speaking or swallowing. Difficulty in breathing, weakness of other muscles, abdominal distension, and constipation may also be common symptoms. People experiencing these problems should seek immediate medical attention.
No illnesses have been reported to date in connection with this problem.
Consumers who have purchased the “Dry Ghoinnya Fish” are urged to return them to the place of purchase for a full refund. Consumers with questions may contact the company at (718) 391-0992.
Pacific International Marketing (“Pacific”) (10/27/2025)
Pacific International Marketing (“Pacific”) is recalling 474 cases of bulk Italian Parsley because it may be contaminated with Salmonella, an organism that can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems.
Healthy persons infected with Salmonella often experience fever, diarrhea (which may be bloody), nausea, vomiting and abdominal pain. In rare circumstances, infection with Salmonella can result in the organism getting into the bloodstream and producing more severe illnesses such as arterial infections (i.e. infected aneurysms), endocarditis and arthritis.
The Italian Parsley was shipped to wholesalers in AZ, CA, FL, MN, MI, GA, OH and NV between September 22 and September 25, 2025. The bulk Parsley was sold in cases as 30 or 60 bunches with a twist tie, and in 24 ct bunches with a twist tie in bags intended for wholesale distribution. The UPC for the 30 and 60 ct bunched Italian Parsley is 40695 80125, located on the twist tie. The UPC for the 24 ct bags is 40695 80120. The shelf life of Italian Parsley is 18 days from harvest, or October 10, 2025.
The company was recently notified that a sample taken on October 6 tested positive. This product should no longer be available directly to the consumer, only if the product is frozen.
Symptoms typically appear 12-72 hours after consuming contaminated food and usually last 4-7 days. In some cases, symptoms may be more severe and require medical attention, especially in young children, older adults, and people with weakened immune systems.
No illnesses have been reported to date.
Pacific is assisting the FDA with its investigation, while concurrently conducting its internal investigation.
Businesses that purchased the recalled product directly from Pacific International Marketing have already been notified. Consumers who have the recalled product in their possession should discard it or return it to the place of purchase for a full refund.
Anyone with questions about this recall may call the company at the above contact number, 8:00 am PST to 5:00 pm PST, Monday through Friday, or by mail to: P.O. Box 3737, Salinas, CA 93912-3737 or email at customerservice@pim4u.com. Updates will also be posted on PIM4U.com. Media inquiries should be directed to Steve Tripp.
E.A. Sween Company (10/27/2025)
E.A. Sween Company, an Eden Prairie, Minn. establishment, is recalling approximately 127,887 pounds of a pulled pork sandwich product that may be contaminated with pieces of plastic, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The barbecue pulled pork sandwich items were produced on various dates between January 13, 2025, and October 15, 2025. The following products are subject to recall [view labels]:
- 5.5-oz. paper wrapped “Deli EXPRESS BBQ PULLED PORK on a sesame bun” sandwiches.
The products subject to recall bear establishment number “EST. 2451” inside the USDA mark of inspection. These items were shipped to retail locations nationwide, including the Department of Defense.
The problem was discovered after the establishment received multiple complaints from consumers finding pieces of plastic in the barbecue pulled pork sandwich product. E.A. Sween Company determined that the plastic originated from the gallon plastic barbecue bottles used in production. There have been no confirmed reports of injury due to consumption of this product. Anyone concerned about an injury should contact a healthcare provider.
FSIS is concerned that some products may be in consumers’ refrigerators or freezers. Consumers who have purchased these products are urged not to consume them. These products should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Consumers and members of the media with questions about the recall can contact the E.A. Sween Company Customer Service Hotline at 1-800-328-8184 and select option #2.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.

LSI, Inc. (10/27/2025)
LSI, Inc., an Alpena, S.D. establishment, is recalling approximately 2,277,540 pounds of a ready-to-eat Korean barbecue pork jerky product that may be contaminated with pieces of metal, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The affected jerky product has a one-year shelf-life with “best by” dates ranging October 23, 2025, through September 23, 2026, printed on the side of the packaging. The following product is subject to recall [view labels]:
- 14.5-oz. and 16-oz. plastic pouches containing “GOLDEN ISLAND fire-grilled PORK JERKY Korean BARBECUE recipe.” A list of the specific product lot codes and best by dates subject to recall can be found here: [view product list].
The product subject to recall bears establishment number “M279A” inside the USDA mark of inspection. This item was shipped to Costco and Sam’s Club retail locations nationwide.
The problem was discovered after the establishment received multiple complaints from consumers finding pieces of wiry metal in the pork jerky product. LSI, Inc. determined that the metal originated from the conveyor belt used in production. There have been no confirmed reports of injury due to consumption of this product. Anyone concerned about an injury should contact a healthcare provider.
FSIS is concerned that some product may be in consumers’ pantries. Consumers who have purchased this product are urged not to consume it. This product should be thrown away or returned to the place of purchase.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Consumers with questions about the recall may contact info@goldenislandjerky.com. Members of the media may contact media@goldenislandjerky.com.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.




Hormel Foods Corporation (10/27/2025)
Hormel Foods Corporation, an Austin, Minn. establishment, is recalling approximately 4,874,815 pounds of foodservice ready-to-eat frozen chicken products that may be contaminated with pieces of metal, the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The affected chicken breast and thigh products were distributed to HRI Commercial Food Service locations nationwide on various dates from February 10, 2025, through September 19, 2025. The following products are subject to recall [view labels]:
- 13.9-lb. cases containing “Hormel FIRE BRAISED MEATS ALL NATURAL BONELESS CHICKEN THIGH MEAT,” with item code “65009” printed on the label.
- 13.8-lb. cases containing 3-oz.“Hormel FIRE BRAISED MEATS ALL NATURAL BONELESS CHICKEN BREAST,” with item code “77531” printed on the label.
- 13.8-lb. cases containing 4-oz.“Hormel FIRE BRAISED MEATS ALL NATURAL BONELESS CHICKEN BREAST,” with item code “46750” printed on the label.
- 23.8-lb. cases containing 5-oz.“Hormel FIRE BRAISED MEATS ALL NATURAL BONELESS CHICKEN BREAST,” with item code “86206” printed on the label.
- 13.95-lb. cases containing “BONELESS CHICKEN BREAST WITH RIB MEAT,” with item code “134394” printed on the label.
A detailed list of the affected pack dates subject to recall can be found here: [view product list]. The products bear establishment number “P-223” inside the USDA mark of inspection.
The problem was discovered after the establishment received multiple complaints from foodservice customers finding metal in their frozen chicken breast and chicken thigh products. Hormel Foods determined that the metal originated from the conveyor belt used in production. There have been no confirmed reports of injury due to consumption of this product. Anyone concerned about an injury should contact a healthcare provider.
FSIS is concerned that some products may be in the freezers of hotels, restaurants and institutions. These businesses are urged not to serve the product. This product should be thrown away.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers.
Consumers with questions about the recall may contact Hormel Foods Customer Relations through their website or by calling 1-800-523-4635. Members of the media may contact media@hormel.com.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.





Teasdale Foods, Inc. (10/27/2025)
Teasdale Foods, Inc. is recalling certain Taco Dinner Kits, because they may contain undeclared milk. People who have an allergy or severe sensitivity to milk run the risk of serious or life-threatening allergic reaction if they consume these products.
Product was distributed to Giant, Martin’s, and Aldi retail stores located in Alabama, Connecticut, District of Columbia, Delaware, Florida, Georgia, Iowa, Illinois, Indiana, Kentucky, Louisiana, Massachusetts, Maryland, Michigan, Mississippi, North Carolina, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, Rhode Island, South Carolina, Tennessee, Virginia, Vermont, Wisconsin and West Virginia.
The potentially affected products are:
- Martin’s and Giant Crunchy Taco Dinner Kit, packaged in a purple box with UPC: 68826757516, with lot code 25257 and Best if used by date: MAR 13 26 printed on the back of the package.
- Casa Mamita Soft Taco Dinner Kit, packaging in a blue and yellow box with UPC: 4099100318715, with lot code 25259 and Best if used by date: MAR 15 26 printed on the back of the package.
No illnesses have been reported to date associated with the implicated product. The recall was initiated after the company received consumer complaints the Taco Dinner Kits contained cocoa mix packets containing milk instead of taco seasoning packets and were distributed in packaging that did not reveal the presence of milk. Subsequent investigation indicates the problem was caused by mislabeling of the taco seasoning packets by the third party that provides the seasoning.
Consumers who have purchased the product are urged to return it to the place of purchase for a full refund. Consumers with questions may contact the company at teasdalecomplaints@teasdalefoods.com.



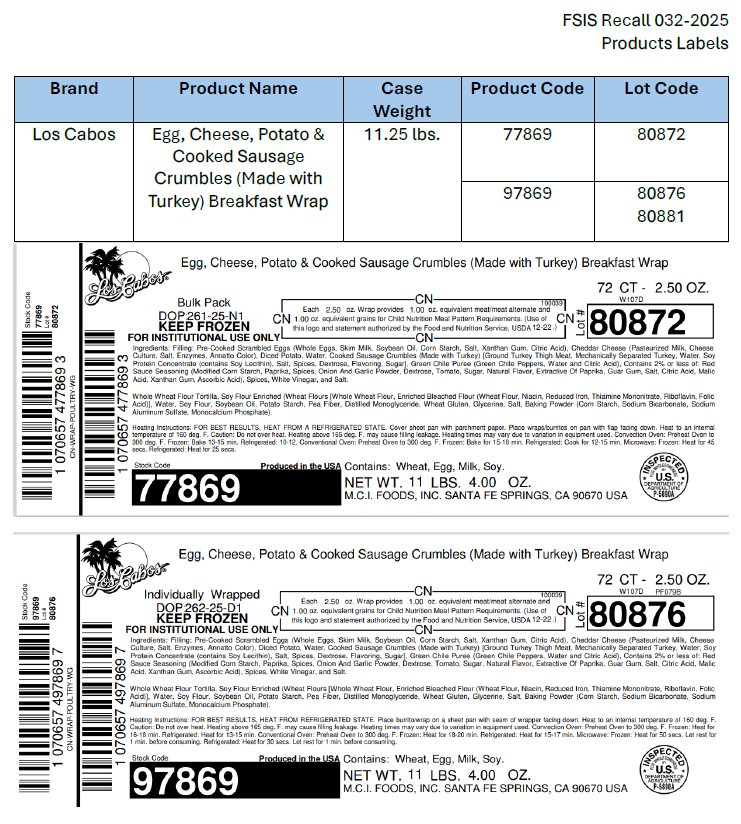
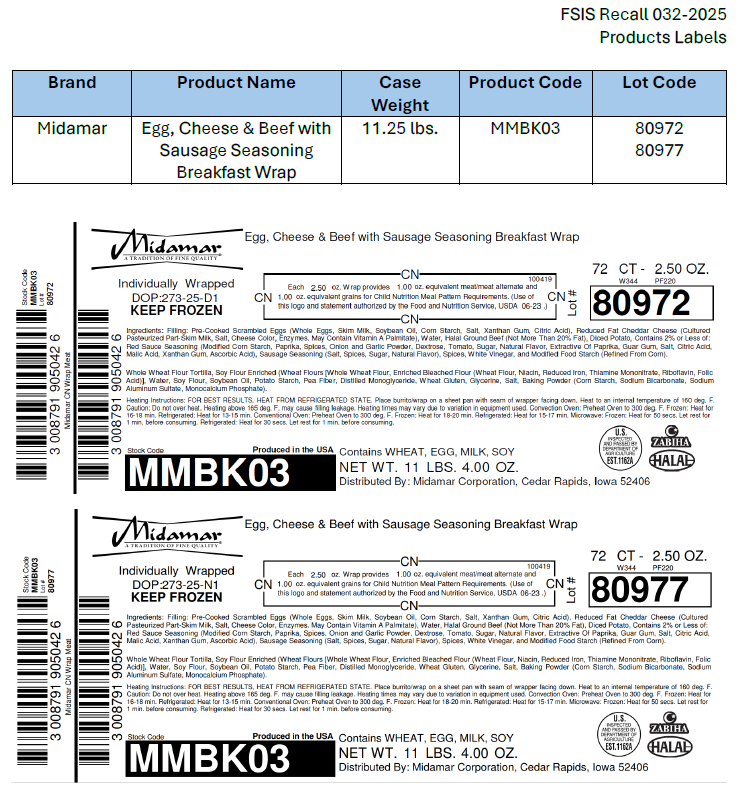
M.C.I. Foods Inc. (10/20/2025)
M.C.I. Foods Inc., a Santa Fe Springs, Calif., establishment, is recalling approximately 91,585 pounds of specific lots of ready-to-eat (RTE) breakfast burrito and wrap products containing egg that may be adulterated with Listeria monocytogenes (Lm), the U.S. Department of Agriculture’s Food Safety and Inspection Service (FSIS) announced today.
The individually packaged and bulk packed frozen breakfast burritos and wraps were produced between September 17, 2025, and October 14, 2025. A list of the products subject to recall can be found here: [view product list]. The labels for the impacted products can be found here: [view labels].
The products bear establishment number “EST. 1162A” or “P-5890A” inside the USDA mark of inspection. These items were shipped to foodservice institutions nationwide. The Los Cabos, El Más Fino and Midamar brand products are included in the USDA’s National School Lunch and Breakfast Programs.
The problem was discovered when the establishment notified FSIS of a positive Lm result in the scrambled egg component after the firm conducted routine sampling and testing of RTE ingredients from its external suppliers.
There have been no confirmed reports of illness due to consumption of these products. Anyone concerned about illness should contact a healthcare provider.
Consumption of food contaminated with Lm can cause listeriosis, a serious infection that primarily affects older adults, persons with weakened immune systems, and pregnant women and their newborns. Less commonly, persons outside these risk groups are affected. Listeriosis can cause fever, muscle aches, headache, stiff neck, confusion, loss of balance and convulsions sometimes preceded by diarrhea or other gastrointestinal symptoms. An invasive infection spreads beyond the gastrointestinal tract. In pregnant women, the infection can cause miscarriages, stillbirths, premature delivery or life-threatening infection of the newborn. In addition, serious and sometimes fatal infections in older adults and persons with weakened immune systems. Listeriosis is treated with antibiotics. Persons in the higher-risk categories who experience flu-like symptoms within two months after eating contaminated food should seek medical care and tell the health care provider about eating the contaminated food.
FSIS is concerned that some product may be in institutional refrigerators or freezers. Institutions are urged not to serve these products. These products should be thrown away.
FSIS routinely conducts recall effectiveness checks to verify recalling firms notify their customers of the recall and that steps are taken to make certain that the product is no longer available to consumers. When available, the retail distribution list(s) will be posted on the FSIS website at www.fsis.usda.gov/recalls.
Media and consumers with questions regarding the recall can contact M.C.I. Foods, Inc. at 888-345-5364.
Consumers with food safety questions can call the toll-free USDA Meat and Poultry Hotline at 888-MPHotline (888-674-6854) or send a question via email to MPHotline@usda.gov. For consumers that need to report a problem with a meat, poultry, or egg product, the online Electronic Consumer Complaint Monitoring System can be accessed 24 hours a day at https://foodcomplaint.fsis.usda.gov/eCCF/.
Haitai, Inc (10/20/2025)
Haitai, Inc of Cerritos, CA is recalling Haetae (HT) brand Cinnamon powder 8 oz because it has the potential to be contaminated with lead.
Short term exposures to very low levels of lead may not elicit any symptoms. It is possible that increased blood lead levels may be the only apparent sign of lead exposure. Exposure to extremely high amounts of lead may result in overt and possibly severe symptoms for which an individual is likely to seek medical attention. While lead can affect nearly every bodily system, its effects depend upon the amount and duration of lead exposure and age/body weight.
If a child is exposed to enough lead for a protracted period of time (e.g., weeks to months) permanent damage to the central nervous system may occur. This can result in learning disorders, developmental defects, and other long-term health problems.
For adults, acute lead poisoning may cause a wide range of symptoms, including abdominal pain, muscle weakness, nausea, vomiting, diarrhea, weight loss, and bloody or decreased urinary output. Chronic lead exposure is associated with kidney dysfunction, hypertension, and neurocognitive effects. Lead can cause serious health problems if too much is ingested, such as damage to the brain and kidneys and can interfere with the production of red blood cells that carry oxygen to all parts of your body.
Product was distributed by Haitai, Inc. and sold at various supermarkets nationwide.
The affected Haetae (HT) Cinnamon is packaged in a square plastic bottle, with medium brown color with undertones of orange and red appearance. Product brand name is Haetae (HT), has a Cinnamon flavor, UPC number 0 20914 81415 9. The recall is for Best by date 02/09/25.
No injuries or illnesses have reported to date. FDA has zero tolerance level for lead for children between 0 to 6 years old. And usually, an adult does not have significant affect from consuming average amount of Cinnamon powder.
The recall was initiated after the FDA collected product samples and detected elevated levels of lead. The firm’s investigation indicates the problem might be caused by potentially adulterated raw material from the supplier or natural lead concentration in Cinnamon and its powder.
Lead poisoning can be diagnosed through clinical testing, and individuals who have consumed affected product should talk to their health care providers about testing.
Consumers who have purchased HT cinnamon powder are urged to return it to the place of purchase for a full refund. Consumers with questions may contact the company at 1-323-890-0101 from 8:00 AM to 5:PM, Monday to Friday days, Pacific time zone.
This recall is being made with the knowledge of the U.S. Food and Drug Administration.
Jody’s Inc. (10/20/2025)
Jody’s Inc. is recalling Cabot Creamery Sea Salt Caramel Cheddar Popcorn 6 oz (170 g) bag, lot number 2519907B1, Best If Enjoyed By: 07 15 26 UPC 8 50016 95530 6, due to presence of undeclared peanuts. People who have an allergy or severe sensitivity to peanuts run the risk of serious or life-threatening allergic reaction if they consume the affected product.
The recalled product was distributed by Jody’s Inc. on July 23, 2025. These products were packaged in foil printed bags, packed in six-pack boxes, and shipped to a distribution warehouse in IL. The product was then further shipped to distribution centers in CA, FL, FL, FL, GA, GA, IL, MA, MD, NC, OR & TX. Product was then shipped to retail facilities across the US.
The recalled product can be identified by the following information:
6oz/170g Cabot Creamery Sea Salt Caramel Cheddar Popcorn
UPC: 8 50016 94430 6
Lot Code: 2519907B1
Expiration Date: July 15, 2026
On October 15, 2025, the firm was notified by The Farmer Companies, Inc. that they received two customers complaints of peanuts found in their bags of the product.
No illnesses have been reported to date.
Consumers who have purchased these products are urged to return them to the place of purchase for a full refund or they may discard the product. Consumers with questions may contact Jody M. Wagner at 757-422-8646 x103, 9:00 AM- 4:30 PM Eastern Time Zone.
This recall is being made with the knowledge of the Food and Drug Administration.